To determine the density of a solid and a liquid and compare the values with their known densities.
The density of a solid and liquid experiment is a classic laboratory experiment. The experiment aims to determine the density of a solid and a liquid and compare the values with their known densities.
The density of a substance is defined as its mass per unit volume:
ρ (g/L) = M / V
In this experiment, the density of a solid and a liquid will be determined and compared with their known densities.
This experiment provides students with hands-on experience in measuring mass and volume and applying the density formula, which is defined as the mass per unit volume of a substance. Also it helps to develop their laboratory skills, including measurement, data analysis, and report writing.

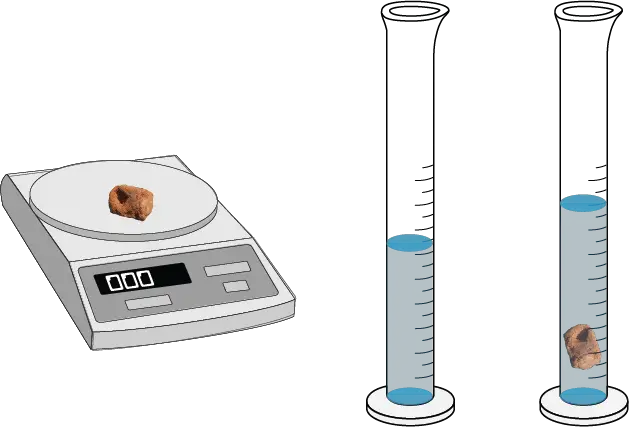
The mass of the solid sample was measured to be “ insert mass “. The volume of the solid was determined to be “ insert volume “. The density of the solid was calculated to be “ insert density “.
The mass of the liquid sample was measured to be [insert mass]. The volume of the liquid was determined to be “ insert volume “. The density of the liquid was calculated to be “ insert density “.
The calculated densities of the solid and liquid were compared with the known densities and found to be “ insert results “.
Insert in the lab report sources of error, such as measurement error, calculation error, or contamination of the sample.
Insert in the lab report suggestions for future work, such as repeating the experiment with a different solid or liquid, or exploring the effect of temperature or pressure on density.
The experiment successfully determined the density of a solid and a liquid and compared the values with their known densities. The results showed that the calculated densities were in agreement with the known densities, indicating that the experimental procedure was accurate and reliable.
The experiment provided hands-on experience in measuring mass and volume, calculating density, and analyzing results, which are important skills for students in the field of chemistry.